Friday 16 October 2015
Wednesday 14 October 2015
Tuesday 13 October 2015
Tuesday 15 September 2015
Organic Chemistry Reaction Scheme
This image shows the key relationships between the functional groups we learn about in this topic. Can you fill in the arrows with suitable reagents? Do you know the importance of the green terms? Why is NH2 underlined in amines? Why is the H underlined in carboxylic acids?
Thursday 10 September 2015
Alcohols - Typical Reactions
In this topic, we will do some experiments with primary, secondary and tertiary alcohols. Here are some videos explaining some of the expected observations and how to use them to identify/classify alcohols.
Alcohols - Preparation from Alkenes
There are a few ways to make alcohols:
- fermentation of sugars
- synthesis gas
- hydration of alkenes
We looked at the hydration of 1-butene using dilute sulfuric acid:
Alcohols - Structure
The structure of alcohols tells us a lot about their physical properties and chemical reactions. Last year, the first lesson was about the different isomers of C4H9OH, with the students starting by trying to make (and name) all of the ones that contain the alcohol functional group. Can you name these?
EXTENSION (not covered in Level 2, but worth seeing if you notice something interesting about the 3D structures of 2-butanol):
Wednesday 9 September 2015
Haloalkanes
Haloalkanes are a very useful intermediate compound, often used when converting one useful compound into another. Their own uses are limited, primarily due to the impact they have on the environment. Haloalkanes are generally immiscible (insoluble in water) as they are (technically) non-polar.
Haloalkanes are the products of:
Haloalkanes are the products of:
- reacting a halogen (such as bromine) with hydrocarbons.
- reacting an alcohol with Lucas Reagent (chloroalkanes produced); remember that tertiary alcohols react very quickly, while primary alcohols may not react at all.
- reacting an alcohol with PCl3, PCl5 or SOCl2
Haloalkanes can be converted into:
- alkenes, using NaOH (or KOH) dissolved in alcohol (elimination reaction)
- alcohols, using aqueous NaOH (or KOH) (substitution reaction)
- amines, using excess NH3 (substitution reaction); if you use do not use enough NH3, it will make an amino salt instead, such as ethyl ammonium chloride
Tuesday 8 September 2015
Identifying Hydrocarbons
Today, we we challenged to work out what class of hydrocarbons were in bottles labelled only as "A", "W" and "Z". Once we had it correct, we were told which hydrocarbons were in each bottle. Here is one way to present our findings if it was an NCEA question:
Alkenes (2)
Here is an introduction to two of the most simple reactions of alkenes: combustion and addition:
Alkenes (1)
Alkenes are unsaturated hydrocarbons. The functional group is a C=C bond (double bond). This lesson is based around the isomers and nomenclature of alkenes:
Here is an activity that shows alkenes have the same general formula as cyclic alkanes:
Here another activity which is just a little tougher:
Wednesday 26 August 2015
Introduction to Organic Chemistry - Alkanes
I'm not a fan of teaching this topic the way Beginning Chemistry does. The following blog posts will be how I taught this in 2014. Hopefully this will complement the work being done in class with our student teacher.
I prefer to "get into it" with the functional groups and cover types of reactions, nomenclature and isomerism over and over again, in context. So, we jumped straight into using MolyMods to make as many isomers of C5H12 as possible.
I prefer to "get into it" with the functional groups and cover types of reactions, nomenclature and isomerism over and over again, in context. So, we jumped straight into using MolyMods to make as many isomers of C5H12 as possible.
Friday 14 August 2015
Wednesday 12 August 2015
Tuesday 11 August 2015
Revision Lesson: Solid Types
Today we did a brief overview of the one of the topics that will be in an Achievement Standard in our upcoming exam:
pH Overview
Today, I taught Mrs Naseem's class and we did an overview of pH and pH Calculations. Here are the whiteboard notes that I gave them. They may be useful to complement the work we did in class:
Friday 7 August 2015
pH of Strong Bases
One of the more difficult skills is calculating the pH of a strong base. To do this, we need to know how to find the hydronium ion concentration from the hydroxide ion concentration:
Acids and Bases Videos
Here are some videos of the key concepts being taught in previous years:
2014
2013
2013
2014
2013
2014
2013
2013
2014
Thursday 6 August 2015
Acids, Bases, Conjugates and Amphiprotic Species
There is a very useful definition of acids and bases worth remembering:
Acids = "proton donors"
Bases = "proton acceptors"
Then, we looked at the pH of some salts and tried to justify the results. For example:
Acids = "proton donors"
Bases = "proton acceptors"
Then, we looked at the pH of some salts and tried to justify the results. For example:
To clarify, ammonium chloride was acidic and sodium ethanoate was alkaline.
Wednesday 5 August 2015
Acid-Base Introduction
This was a short overview of the acid-base aspect of the topic. There are a few new things added to what we were expected to know from last year:
- How to calculate pH from the hydrogen (actually hydronium) ion concentration.
- How to calculate the hydronium ion concentration from the pH
- How to link pH, hydronium ion concentration and properties, such as rate of reaction
We also found out a couple of "lies" from last year:
Friday 31 July 2015
Equilibrium Constant and Industrial Equilibrium
The details of these concepts can be found on pp209-211. Our homework question also explores these ideas.
Wednesday 29 July 2015
Wednesday 22 July 2015
Rate of Reaction
This week, we are exploring the factors that may affect reaction rate:
Each group is challenged to create a presentation (video, PowerPoint, report...) to explain how their respective factor affects reaction rate. The presentations should include models and/or experiments that give a visual aid to understanding the key concepts. This is due on Tuesday, next week.
Parallel to this learning, we have an old NCEA question to complete for homework. This is due on Monday night, via Moodle.
Each group is challenged to create a presentation (video, PowerPoint, report...) to explain how their respective factor affects reaction rate. The presentations should include models and/or experiments that give a visual aid to understanding the key concepts. This is due on Tuesday, next week.
Parallel to this learning, we have an old NCEA question to complete for homework. This is due on Monday night, via Moodle.
Wednesday 24 June 2015
Identifying Species
When we do redox reactions, sometimes it is difficult/uncertain which species have been produced. We have other chemical tests to help us confirm which species (ions/molecules) are present. We do not need to write equations for these in the assessment for this topic, but they are worth knowing anyway...
One species not mentioned here is copper (II), Cu2+:
Adding NaOH:
Cu2+ + 2OH- --> Cu(OH)2
This is a blue precipitate.
Adding excess NH3 solution:
Cu2+ + 4NH3 <--> [Cu(NH3)4]2+
This is a royal blue solution.
One species not mentioned here is copper (II), Cu2+:
Adding NaOH:
Cu2+ + 2OH- --> Cu(OH)2
This is a blue precipitate.
Adding excess NH3 solution:
Cu2+ + 4NH3 <--> [Cu(NH3)4]2+
This is a royal blue solution.
Wednesday 17 June 2015
Redox Reactions
Over the next few lessons, we will be carrying out some redox reactions and justifying why they are indeed redox. This is very similar to the assessment task in the last week of term.
Monday 8 June 2015
Friday 5 June 2015
Redox Tutorials
Mr Nicoll is away for the week, so the following videos have been made available to help with the work in class:
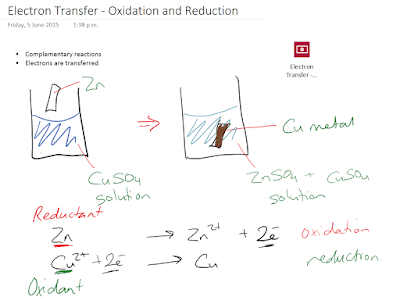
Electron Transfer
Oxidation-reduction is another class of reaction that we need to know about. What is oxidation-reduction? It is a reaction where electrons are transferred.
Oxidation and Reduction
Oxidation and reduction are complementary processes. When one occurs, the other must also occur.
Oxidation Numbers
One way to determine whether oxidation-reduction has occurred or not is to see if the oxidation number of a species has changed. Oxidation numbers also tell us what has been oxidised and what has been reduced.
Electron Transfer
Oxidation-reduction is another class of reaction that we need to know about. What is oxidation-reduction? It is a reaction where electrons are transferred.
Oxidation and Reduction
Oxidation and reduction are complementary processes. When one occurs, the other must also occur.
Oxidation Numbers
One way to determine whether oxidation-reduction has occurred or not is to see if the oxidation number of a species has changed. Oxidation numbers also tell us what has been oxidised and what has been reduced.
Thursday 28 May 2015
Bond Energies
It requires energy to break chemical bonds. Conversely, energy is released when bonds are made. The difference between these values tells us whether a reaction is endothermic (positive) or exothermic (negative).
2014 Video:
2014 Video:
2013 Video:
Tuesday 26 May 2015
Thursday 21 May 2015
Thursday 14 May 2015
Molecule Polarity
This was just an introduction to polarity. We will be exploring this more in the next lesson.
Our task was to determine the polarity of the molecules we did ball-and-stick diagrams for in yesterday's lesson.
Here are a couple of observations we need to be able to explain, using molecular polarity:
Our task was to determine the polarity of the molecules we did ball-and-stick diagrams for in yesterday's lesson.
Here are a couple of observations we need to be able to explain, using molecular polarity:
- Polar solutes dissolve better in polar solvents. Non-polar solutes are only sparingly soluble in polar solvents. Why? The example we were given was ammonia (NH3) dissolving in water.
SOURCE: UC Davis ChemWiki - Polar molecules have higher melting and boiling points than non-polar molecules with a similar-sized electron cloud. For example, methane (MR = 16.0 gmol-1) is a gas at room temperature while water (MR = 18.0 gmol-1) is a liquid. Why?
SOURCE: http://chemsite.lsrhs.net/bonding/lewis_dots.html
 |
| SOURCE: http://www.ausetute.com.au/lewisstr.html |
Subscribe to:
Posts (Atom)